Manufacturing quality does not fail because teams ignore controls. It fails when risk identification and process control are not aligned.
PFMEA and Control Plans exist to bridge that gap. However, many organizations still treat them as similar documents or rely on one to cover weaknesses in the other. This misunderstanding creates hidden risk, audit findings, and unstable processes that only surface under pressure.
This article explains the proper role of PFMEA and Control Plans, how they differ, and how quality standards expect them to work together to prevent failures and maintain consistent process performance.
What Is PFMEA (Process Failure Mode and Effects Analysis)?
PFMEA is a proactive risk analysis method used to evaluate how a manufacturing or assembly process could fail, why that failure might occur, and what the impact would be if it did.
PFMEA focuses on potential failures, not defects that have already happened.
Purpose of PFMEA
PFMEA helps teams:
- Identify possible failure modes at each process step
- Understand the effects of those failures on the customer or downstream operations
- Analyze causes and estimate how often failures could occur
- Evaluate how effectively current controls can detect failures
- Prioritize actions using Action Priority or Risk Priority Number
The objective is clear. PFMEA reduces risk before problems reach production or customers.
When PFMEA Is Required
Under the AIAG–VDA FMEA methodology, organizations must create or update PFMEA when:
- Introducing a new manufacturing or assembly process
- Changing equipment, tooling, materials, or suppliers
- Relocating or rebalancing a process
- Investigating field failures or recurring internal issues
Teams must complete PFMEA before finalizing control strategies. Performing PFMEA after controls are already set defeats its purpose.
What PFMEA Is Not
PFMEA is not:
- A defect log
- A response to customer complaints
- A control document
- A one-time exercise
If PFMEA does not change as the process evolves, it no longer represents real risk.
What Is a Control Plan?
A Control Plan defines how critical process and product characteristics are controlled during production.
It translates risk understanding into daily execution on the shop floor.
While PFMEA identifies where risk exists, the Control Plan ensures that those risks remain controlled once production starts.
Purpose of a Control Plan
A Control Plan ensures that:
- Key characteristics stay within specification
- Variation is detected early
- Operators know precisely how to react to deviations
- The process produces consistent output over time
The Control Plan does not analyze risk. It manages process behavior in real time.
When Control Plans Are Used
Organizations develop Control Plans:
- After PFMEA identifies significant risks
- During process validation and launch
- Throughout the entire production lifecycle
Control Plans must remain living documents that reflect how the process actually runs, not how it was designed years ago.
What a Control Plan Includes
A technically correct Control Plan defines:
- Process step
- Controlled characteristic
- Specification or tolerance
- Control or measurement method
- Sampling size and frequency
- Responsible owner
- Reaction plan for out-of-control conditions
A Control Plan without a clear reaction plan does not meet intent, even if measurements exist.
PFMEA vs Control Plan: Key Differences
PFMEA and Control Plans answer different questions.
PFMEA asks:
- What could go wrong?
- Why could it happen?
- How serious would the impact be?
The Control Plan asks:
- How do we monitor this characteristic?
- How often do we check it?
- What actions do we take when it is out of control?
Technical Comparison
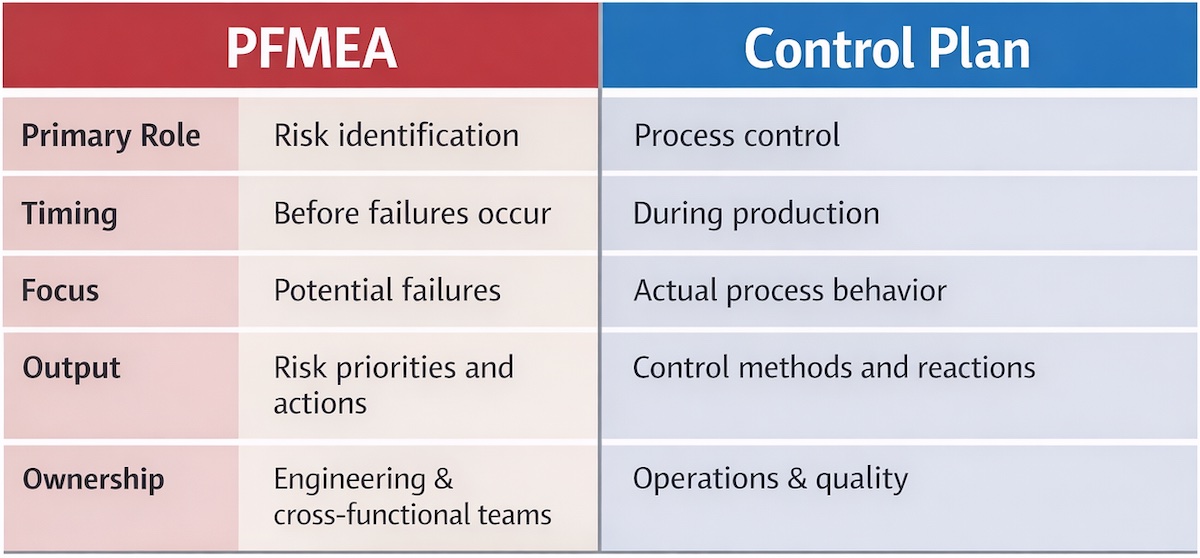
When teams try to make one document perform both roles, they weaken either the analysis or the control.
How PFMEA and Control Plans Work Together
PFMEA and Control Plans form a closed-loop system.
PFMEA Drives the Control Plan
High-risk failure modes identified in PFMEA must appear in the Control Plan as:
- Controlled characteristics
- Appropriate control methods
- Sampling frequencies that reflect actual risk
- Clear and enforceable reaction steps
If PFMEA identifies a high-severity failure but the Control Plan doesn’t address the associated Product or Process Characteristic of that failure, the system is misaligned.
Control Plans Validate PFMEA
Production data often reveals:
- Occurrence assumptions that no longer hold
- Detection controls that are less effective than expected
- New failure modes introduced by real operating conditions
Teams must feed this information back into PFMEA updates. This feedback loop is a core expectation of AIAG–VDA FMEA.
Why Control Plans Without PFMEA Create Risk
Organizations that build Control Plans without PFMEA commonly:
- Over-control low-risk characteristics
- Miss high-impact failures
- Apply inconsistent or ineffective reaction logic
- Increase inspection cost without reducing real risk
From an audit perspective, a missing linkage between PFMEA and Control Plans signals weak risk-based thinking rather than simple documentation gaps.
Practical Manufacturing Example
Consider a press-fit operation.
PFMEA Identifies
- Failure mode: Insufficient press depth
- Effect: Loss of function during customer use
- Cause: Tool wear, misalignment, material variation
- Severity: High
- Occurrence: Medium
- Detection: Low without in-process monitoring
Control Plan Implements
- Controlled characteristic: Press depth
- Frequency: 100 percent monitoring
- Control method: In-process displacement sensor measurement data – SPC analysis
- Reaction plan: Stop production, inspect tooling, and segregate affected parts
Without PFMEA, teams might still measure press depth, but not with the correct urgency, frequency, or reaction discipline. Without the SPC analysis control method, the process might stay within limits, but be wildly out of control and producing high process variability.
When to Update PFMEA vs Control Plans
Update PFMEA When
- The process changes
- Failures occur internally or in the field
- New risks become visible
- Control effectiveness changes significantly
PFMEA updates are analytical and strategic.
Update Control Plans When
- Measurement methods improve
- Process capability improves
- Sampling strategies change
- Operator feedback reveals execution gaps
Control Plan updates are operational and tactical.
Conclusion: Linking Risk Prevention to Process Control
PFMEA and Control Plans serve different purposes, but they only deliver value when teams link them consistently and keep them current.
PFMEA identifies and prioritizes risk before production begins. Control Plans ensure those risks remain controlled once the process is running. When organizations maintain a clear connection between the two, quality becomes predictable rather than reactive.
At APiS North America®, we support this approach by treating PFMEA and Control Plans as integrated elements of one risk management system, not isolated documents.
With APIS® IQ-Software, teams can maintain AIAG–VDA-aligned PFMEAs, preserve traceability between failure modes and controls, and keep both documents accurate as processes evolve.
The result is not better paperwork. It is clearer risk understanding, stronger execution, and more reliable manufacturing processes.